Preventing M. hyo and PCV2 to control PRDC
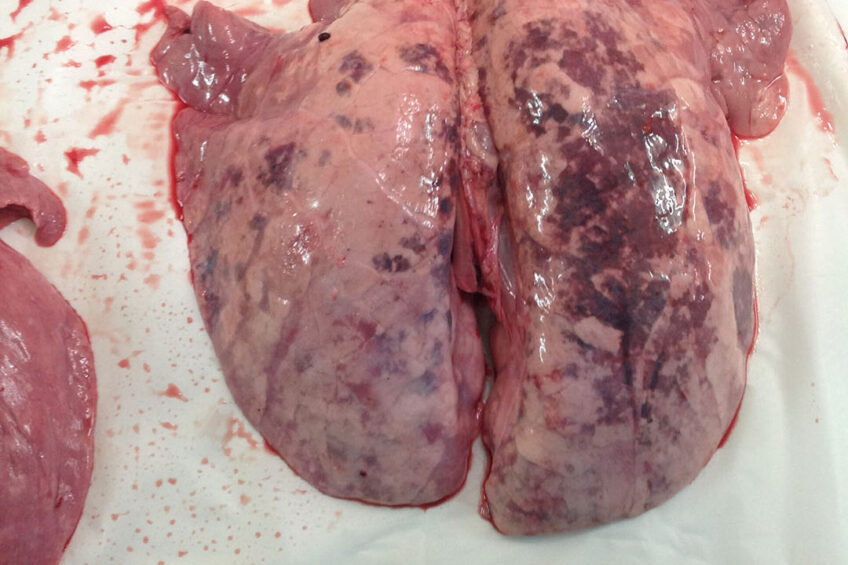
As knowledge grows of lung health problems in pigs, it is increasingly clear that porcine circovirus 2 is playing an important role in the severity of Porcine Respiratory Disease Complex. The virus is often present in co-infections with other pathogens, especially Mycoplasma hyopneumoniae.
Porcine Respiratory Disease Complex (PRDC) causes substantial economic losses in the swine industry due to decreased growth rate, increased feed conversion ratio, increased treatment costs and increased mortality. Morbidity rates associated with PRDC may range from 30% to 70%, and mortality rates may be between 4% and 6% or even higher in affected farms.
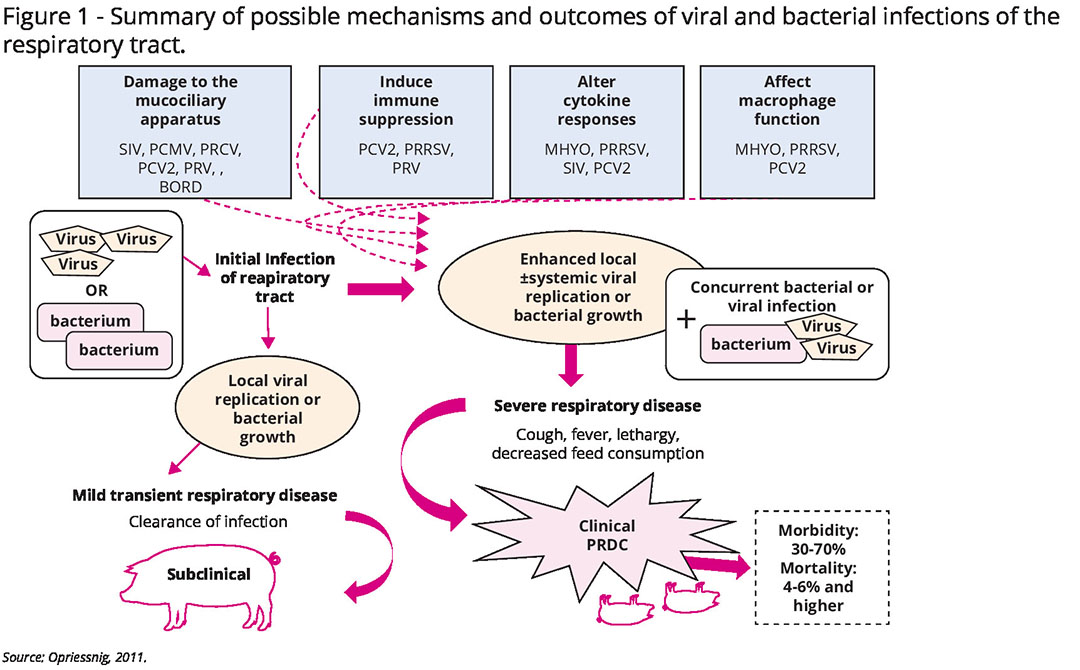
PRDC develops as a consequence of coinfections of multiple primary viral and bacterial micro-organisms; apart from Mycoplasma hyopneumoniae (M. hyo), viruses like porcine circovirus type 2 (PCV2), Porcine Reproductive and Respiratory Syndrome virus (PRRSv) and swine influenza virus are the most frequently found.
Other viral pathogens – Aujeszky’s disease virus, porcine respiratory coronavirus (PRCv) – and bacteria Actinobacillus pleuropneumoniae (App) or Streptococcus suis may be involved. Pasteurella multocida and Glaesserella parasuis are the most frequent secondary bacteria, complicating pneumonia. However, as a true multifactorial disease, environmental conditions, population size, management strategies and pig-specific factors such as age and breed also play critical roles in the outcome of PRDC.
Role of PCV2 in PRDC
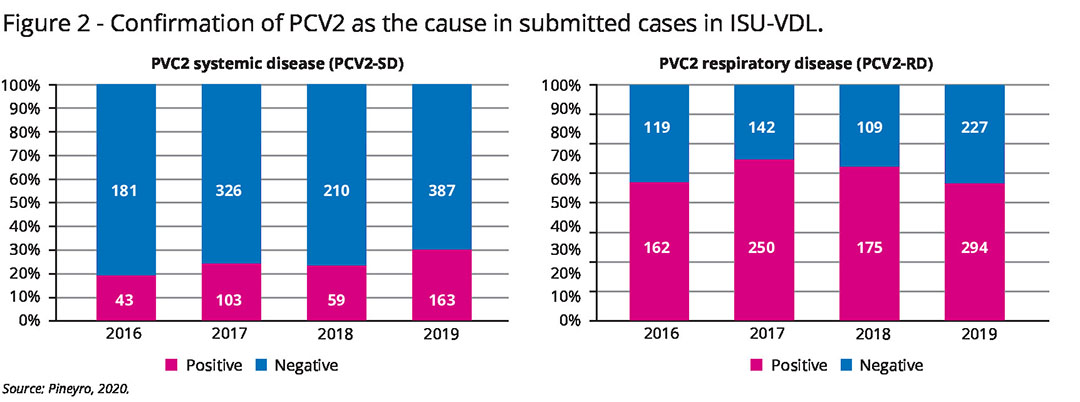
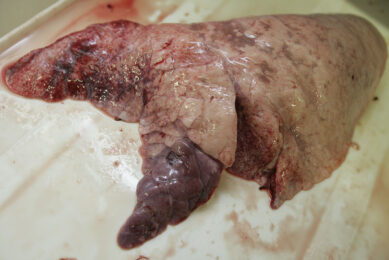
PCV2 is now widely accepted to be an important pathogen in PRDC. PCV2 antigens have been demonstrated in the cytoplasm of pulmonary macrophages, the bronchiolar epithelium and endothelial cells, which suggests that PCV2 may contribute to the severity and duration of PRDC. PCV2 is frequently confirmed as a cause of respiratory disease (see Figure 2).
PCV2 can cause respiratory lesions in the absence of systemic lymphoid damage; that specific condition has been referred to as PCV2 lung disease (PCV2-LD). PCV2-LD is diagnosed when clinical signs like respiratory distress or dyspnoea are present, characteristic pulmonary lesions are observed in the absence of lymphoid lesions and a moderate to high amount of PCV2 is detected in the lungs but there is a lack of virus in lymphoid tissues. However, it was demonstrated that PCV2-LD is probably a negligible condition in the field, and PCV2 mainly contributes to PRDC in relation to PCV2 systemic disease occurrence.
PCV2 is determined to be associated with PRDC when PCV2 antigen is associated with characteristic lung lesions. Those lesions include necrotising and ulcerative bronchiolitis, granulomatous inflammation in the alveolar septa and mixed inflammation and fibroplasias in the lamina propria and peribronchiolar areas.
Co-infection with other pathogens
A co-infection of PCV2 with another bacterial pathogen is frequently diagnosed in PRDC. In a retrospective study describing the association of PCV2 with PRDC, PCV2 was found in 81%, the combination of PCV2 and P. multocida in 36%, followed by PCV2 and M. hyo in 31% cases. The results of that survey indicate that PCV2 is widely prevalent in pigs with PRDC and should be considered a major respiratory pathogen.
Implementation of PCV2 vaccination in PRDC-affected herds substantially lowers the incidence of respiratory disease and pulmonary co-infections. A pig vaccinated against PCV2 from a fattening farm had only half the chance (OR 0.51) of pneumonia being detected at post-mortem than a non-vaccinated pig from a farrow-to-finish farm (see Table 1). This study demonstrated the benefit of a vaccination programme against PCV2 as an important tool to reduce the risk of post-mortem pneumonia findings and the severity of pneumonia in pigs at slaughter.
Role of M. hyo in PRDC
M. hyo is the primary pathogen of enzootic pneumonia and the most important bacterial primary agent involved in PRDC. M. hyo participates in lung damage by itself through the degeneration of bronchial cilia and over-reaction of the local immune system in lungs, resulting in a strong inflammatory response. The destruction of cilia leads to the dysfunction of the mucociliary defence system. Loss of the mucociliary apparatus and ineffective mucosal clearance also allows increased colonisation by secondary bacteria such as P. multocida and others. It was also described that the efficiency of phagocytic cells in lungs can be suppressed during M. hyo infection. M. hyo diminishes the ability of the respiratory tract to respond to other pathogens and increases the severity and duration of respiratory disease induced by other pathogens.
Although M. hyo infects different target cells, PCV2 and M. hyo co-infected pigs show more severe clinical respiratory disease and lung lesions, poorer growth performance, longer PCV2 viraemia and greater amounts of PCV2 antigen in serum, lymphoid and lung tissues than pigs infected with only one pathogen. The peribronchial lymphoid hyperplasia induced by M. hyo seems to provide an important site for PCV2 replication in the lung.
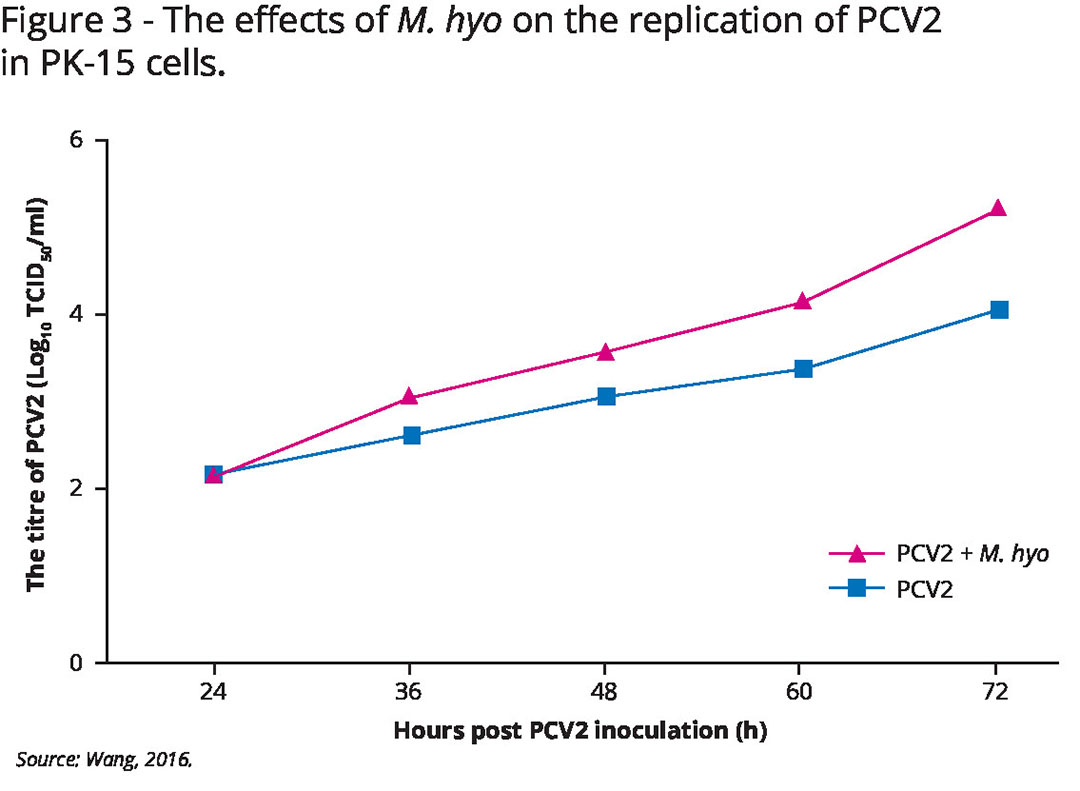
M. hyo was proven to enhance the replication of PCV2 in a time- and dose-dependent manner. Furthermore, different magnitudes of PCV2-infected cells were detected when co-infected with different M. hyo strains (see Figure 3 and Table 2).
In vivo study of PCV2 and M. hyo
In an in vivo study, pigs inoculated firstly with M. hyo and 2 weeks later with PCV2 had increased PCV2-associated lesion severity. That increased severity was attributed to the ability of M. hyo to up-regulate macrophage proliferation and, therefore, to facilitate PCV2 replication, together with an alteration of cytokine production.
M. hyo also potentiated differently the severity of consequent PCV2 infection depending on PCV2 genotype. Dual infection with 2 pathogens produced more severe respiratory scores (highest for M. hyo + PCV2d), and reduced weight gain in pigs compared with pigs only inoculated with PCV2. The overall levels of PCV2d viraemia and severity of lymphoid lesions were significantly higher in pigs dually inoculated with M. hyo/PCV2d when compared with all other dually inoculated groups. The results of that study demonstrated that M. hyo potentiated the replication of PCV2d more than it did with the other PCV2 genotypes. Those results indicated that synergistic effects occur during the process of M. hyo and PCV2 sequential infection in experiments and also in the field condition.
Vaccination against M. hyo significantly protected pigs against multiple viral infections, suggesting that vaccination against M. hyo decreases the risk of PRDC and reduces susceptibility of pigs to other viral pathogens.
Conclusion
The above-mentioned results of the extensive research demonstrated the pivotal role of both M. hyo and PCV2 in the development of PRDC. They act directly by causing different types of pneumonia and indirectly by affecting the defence mechanisms, facilitating other viral or bacterial infections. Efficient control of those infections is essential to protect the integrity of lung tissue and maintain functionality of the local immune system so that it can combat other co-infections.
Respiratory health can then be monitored in slaughter pigs via lung lesion scoring. At the same time, other infections that may aggravate the course of respiratory disease – such as PRRSv, swine influenza virus or P. multocida – must be watched carefully. That way differential diagnosis can be done properly, other infections can be controlled and disturbance can be avoided through PCV2 and M. hyo post-vaccine immunity.
References available upon request.
 Beheer
Beheer



 WP Admin
WP Admin  Bewerk bericht
Bewerk bericht