It´s not all about the strains
There are many factors to consider if an FMD vaccine is “good.” Not only the quality of a highly purified FMD vaccine impacts on its safety and efficacy but also, it allows to differentiate infected from vaccinated animals (DIVA). In this article, let’s focus on another important piece of a high-quality FMD vaccine: the vaccine strain.
There is a popular concept that states that a high-quality vaccine must contain the latest strain isolated from the same country or region where the vaccine will be administered. But is that truly necessary? The answer to this question is: NO, it´s not!
Key points to consider
So, what are the key points that need to be considered when we evaluate a vaccine strain? It is crucial to know the level of cross-protection that the vaccine can reach against the circulating virus in the region. Where can we check this information? Some of the FMD vaccines in the market are continuously tested against the latest isolates in every region by tests performed by FMD World Reference Lab with the results published in their quarterly reports. Also, there are more complex potency tests, that require vaccinated animals to be challenged with a virulent FMD virus; such as PD50 or PGP test.
However, vaccine strains are not alone in this war against viruses. It’s not all about the strain, it is also about how the vaccine is manufactured and formulated. For instance, the nature of the adjuvant will determine how long the immunity might last, the production process will influence the safety of the vaccine and quality control points will make sure the product is good to market.
O1 Campos
Let´s bring the example of a well-known FMD vaccine strain such as O1 Campos. O1 Campos was first isolated back in 1958 in South America and later incorporated into several different products. Now, does it behave in the same way? Are all O1 Campos the same? NO!
O1 Campos Biogenesis Bago has been able to control FMD O serotype outbreaks all around the world for several years now. It is continuously challenged against the latest isolations and does not seize to amaze with its results (Figure 1). So, what makes Biogenesis Bago’s O1 Campos so special? It’s the whole package!
Figure 1- O1 Campos high potency vaccine heterologous neutralisation titers against type O field isolates.
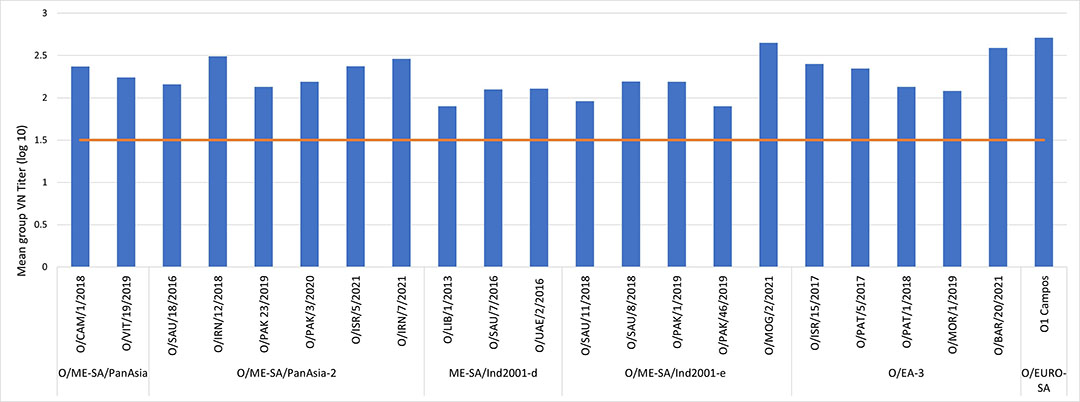
For instance, back in 1997 in Taiwan, O1 Campos (Biogenesis Bago) was instrumental during the outbreak control until the country successfully regained its status of being free from FMD without vaccination in 2020. That means they were able to start exporting pork products again.
In Argentina, during the outbreaks of 2000, when the country was free without vaccination, Biogenesis Bago’s FMD vaccines helped control the outbreak within 11 months, with two vaccination rounds, delivering and administering 120 million doses in 6 months.
The situation in South Korea and Vietnam was different. Even with vaccination, controlling the disease was not an easy task. However, once Aftogen Oleo and Bioaftogen became available, there was a new and potent tool for fighting the disease. Currently, free of outbreaks, South Korea applied at WOAH to be a country free of FMD with vaccination.
And finally, just this year, Indonesia, Israel, and Palestine started using Biogenesis Bago’s vaccines to help control and prevent the further spread of FMD in their territories.
Offering solutions to farmers, vets and health authorities
The data from FMD World Reference labs and actual field experiences across the globe support the broad cross-protection claim of O1 Campos of Biogenesis Bago. And, at the end of the day, to control and eradicate FMD, this is what we need: World- class vaccines with strains strong enough to offer real solutions to farmers, veterinarians and health authorities.
So, next time you have to choose a vaccine, remember, it´s not just about the strain; it´s everything else that comes with it: state-of-the-art manufacturing process, quality controls and certifications, proven experience around the world, and technical and scientific support. It´s about choosing a World-Class FMD Vaccine!
 Beheer
Beheer




 WP Admin
WP Admin  Bewerk bericht
Bewerk bericht