Inactivating ASFv: Does it require 59°C or 100°C?
With African Swine Fever virus (ASFv) concerns continuing to weigh heavily on the swine industry, at times it can be difficult to interpret and reconcile opposing research results. A recent example of this involves separate studies related to the temperature required for inactivating ASFv to prevent transmission.
Time and temperature are currently the best ways to inactivate ASFv. Different methods to evaluate both time and temperature, however, can lead to different outcomes.
2 papers, 2 different conclusions on inactivating ASFv
In 2023, the University of Minnesota (UMN) released preprint data indicating that 2 double-stranded, multilayered membrane-enveloped DNA mega-viruses, Emiliania huxleyi virus (EhV) and ASF virus, were damaged by heat treatment but remained potentially viable when exposed to temperatures up to 100°C for 20 minutes. However, in a published journal article in Pathogens, also published in 2023, the German ASFv reference lab reported that 59°C effectively eliminated infectivity of ASFv.
Why do results from these 2 papers have vastly different conclusions? The main difference is based on the methods used to evaluate the potential infective capacity of ASFv subjected to different heat treatments.
UMN: Viable PCR method
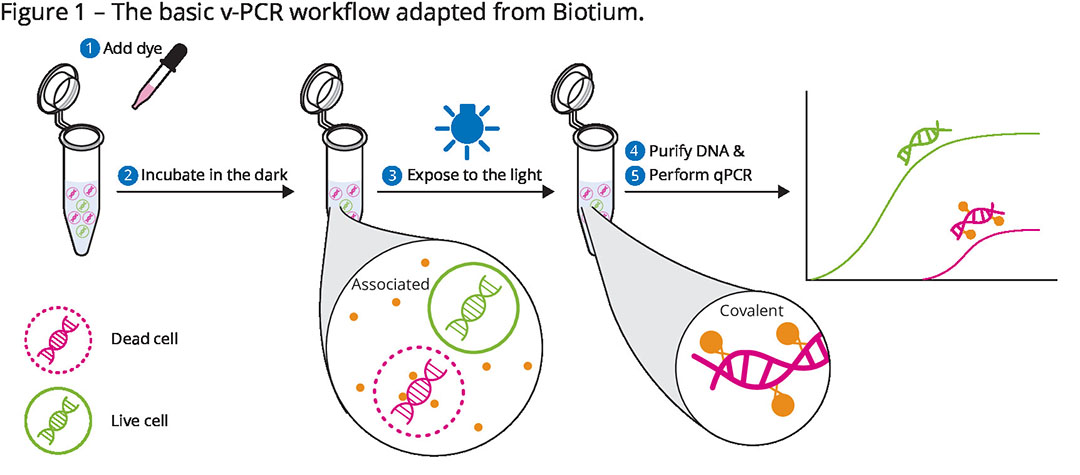
UMN used a “viable” PCR method, which uses a special dye to penetrate the membrane of the mega-viruses damaged by heat treatment (see Figure 1). The theory is that the dye can penetrate damaged membrane and bind with the DNA genome, thus interfering with PCR replication. However, the dye does not penetrate undamaged membranes. Therefore, the viable PCR method described by UMN can distinguish between intact membrane virus particles versus damaged membrane virus particles, which is an advantage over standard PCR methods that only detect genome regardless of the degree of membrane damage.
No further replication?
The discovery of limited dye penetration in both EhV and ASFv, even when exposed to temperatures up to 100°C for 20 minutes, is an interesting observation that needs further research. However, this does not necessarily mean the virus particles are still capable of causing infection. Even if the dye does not penetrate undamaged membranes, this does not mean that the treatment did not damage virus genome and render it incapable of further replication. For example, ultraviolet light (UV-C) exposure damages genome cross-linking, rendering a virus incapable of replication but not necessarily damaging the virus membrane.
This aspect of the viable PCR method to equate the resistance to dye penetration with the ability of the virus particle to infect an animal cell has not been scientifically validated for ASFv. In fact, in this same publication by UMN the authors also evaluated the infectivity of EhV in cell culture, and exposure to 50°C and 60°C was sufficient to eliminate viral infection after 30 seconds.
German ASFv reference lab
The German ASFv reference lab used the historically widely accepted method to examine the infectivity of ASFv after composting infected wild boar carcasses. That approach is a scientifically validated method for evaluating ASFv infectivity using a virus isolation method with porcine peripheral blood-derived macrophages.
The primary mechanism of infection by ASFv is through phagocytosis by porcine macrophage cells. Exposing ASFv to their target macrophage cells in culture provides phagocytosis conditions that mimic a natural infection. The study showed that ASFv was no longer infectious once the compost pile temperature reached 59°C, although the ASFv genome using the standard PCR method remained present, again confirming that standard PCR methods do not distinguish between viable and non-viable virus.
Considering the limited membrane damage UMN reported, the loss of infectivity at 59°C suggests that part of the ASFv genome or key components of membranes were damaged, rendering it incapable of infection. The thermal inactivation data from the German ASFv reference lab confirms results of previous studies that 60°C for 30 minutes and 60°C for 15–20 minutes was sufficient to inactivate ASFv infectivity. Also, the EU directive 2002/99/EC recognises that heat treatment at a minimum temperature of 80°C is sufficient to inactivate ASFv in meat.
Potential viable genome vs infectivity
A potential viable genome does not equal infectivity. It is well known that DNA is very stable at temperatures below 100°C. PCR tests depend on the fact that DNA remains stable at 95°C. Therefore, it is not surprising that PCR tests can detect the ASFv genome even after 100°C heat treatment. Limited dye penetration even when both EhV and ASFv were exposed to temperatures up to 100°C for 20 minutes is a very interesting discovery. These results suggest that viable genome could potentially remain even with high-temperature treatment.
In fact, both EhV and ASFv lost their infectivity with 50°C and 60°C heat treatments, as reported by both the UMN and the Germany ASFv reference lab paper, as well as in previous publications. Both studies demonstrated that heat treatments were effective in reducing infectivity. Other methods such as viral exposure to UV-C could provide additional assurance for inactivating hardy viruses like ASFv.
Further development and scientific validation necessary
Further development and scientific validation of the viable PCR method as an indicator of viable virus is necessary before this technology should be used to provide appropriate temperature and/or chemical treatment guidelines to inactivate ASFv.
 Beheer
Beheer







 WP Admin
WP Admin  Bewerk bericht
Bewerk bericht