Questions and answers around sow claw trimming
Lameness is a multifactorial problem, including nutrition, poor horn quality, trauma, infection, type of housing and walking surface. This paper reviews the functional morphology of the critical structures involved in horn formation and growth to better understand the mechanisms involved in the development of lameness.
By Dr Sarel Van Amstel, professor of farm animal medicine and surgery, University of Tennessee, USA
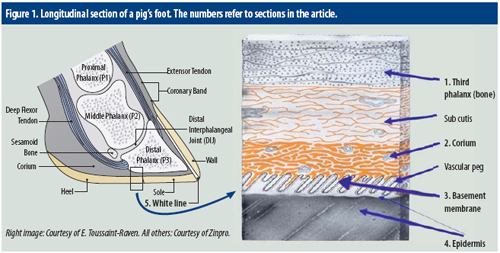
Pig producers themselves can help to prevent sow lameness problems, by trimming claw problems when noted. What, however, is going on inside a sow’s claw when it needs trimming? Essential structures in a sow’s claw are demonstrated in Figure 1. Critical structures involved in horn formation and growth include the corium; basement membrane; and germinal layers of the epidermis. Working from inside out, the approach first zooms in on the bone in the claw, called the third phalanx. Some explaining is required for the white line, a special, visible zone in a claw.
1. Third (distal) phalanx
The bone in the claw is also referred to as the third phalanx (P3). The first and second phalanx are located higher up in the pig’s foot. The third phalanx is suspended in the claw horn capsule through the subcutis. The third phalanx has a number of functions:
• It transmits the weight bearing forcesassociated with standing and walking through the corium and the wall to the outside.
• It serves as an anchor for the large tendons (extensors in the front and flexors at the back) which are responsible for flexing and extending the foot during locomotion. The third phalanx is not often involved in lameness as it is fairly well protected within the claw capsule. However, deep penetrations through the heel, white line, sole or coronary band may spread infection into the bone resulting in severe lameness.
2. Corium
The corium is situated between the third phalanx and the horn capsule and is a highly innervated and vascular structure responsible for nutrient and oxygen supply to the proliferating and differentiating layers of the horn producing layer, also known as the epidermis.
Claw lesions involving the corium lead to pain and lameness. In addition, compromised blood flow through the corium, such as caused by inflammation due to trauma, infection or inappropriate nutrition will interfere with horn production and may manifest as horizontal or vertical wall cracks, erosions and ulcers in the sole or heel as well as white line separation.
3. Basement membrane
The basement membrane is situated between the corium and inner layer of the epidermis. The basement membrane contains a complex lattice work of elastic fibres which attaches the basal (inner) layer of epidermis to the basement membrane. The basement membrane is the key structure bridging the epidermis via the corium to the connective tissue of the third phalanx and as such provides epidermal anchorage and orientation. Damage of the basement membrane leads to loss of organised structure including:
1. Tubular and non-tubular horn architecture (see below); and
2. Proliferation and differentiation of the horn in the epidermis with abnormal pattern and expression of keratin (internal cell structure) and poor horn quality (soft horn).
4. Epidermis
Horn develops from the epidermis which is the layer below the corium and basement membrane. Horn consists of two different types, called tubular and intertubular horn.
Tubular horn
The epidermal layer overlying the vascular pegs (projections from the surface of the corium, Figure 1 ) produces horn cells in the form of tubules (tubular horn). Tubules differ in size, number and shape in various parts of the claw and are round near the inside of the wall and oval near the surface. There are more tubules per mm 2 in the wall as compared to the sole and heel.
Intertubular horn
Intertubular horn is produced between the vascular pegs and interconnects the tubular horn. Intertubular horn consists of sheets of elongated cells arranged parallel with the bearing surface. Since tubular horn is what imparts structural strength to the horn capsule, it follows that the horn of the wall is the strongest followed by the sole and the heel. Horn cells become large and flatter as they move from the base of the epidermis to the outside where they undergo programmed death and replacement of cell contents by keratin proteins.
Keratin
Keratin is a fibre-reinforced composite material and is one of toughest bio-materials. Keratin consists of long, slender fibers aligned parallel to the long axis of the cell. It acts as internal scaffolding giving the cell strength and rigidity. The strength of keratin is dependent on adequate trace minerals such as copper and zinc. Biotin may also play a role particularly with the cementing substance which serves to connect tubular and intertubular horn cells. The cell membrane of horn cells on the surface remains permeable to water thus excessive moisture can lead to soft horn and increased wear, causing lameness.
5. White line
The white line connects the sole to the wall and consists of soft flexible horn. It is very susceptible to the abrasive effect of concrete and the erosive effects of bacterial enzymes. Because of the soft horn, cell turn-over in the white line is faster than in other parts of the claw with the result that more immature cells are in contact with the bearing surface. White line separation is also linked to the biomechanics of weight bearing (the way the animal walks). The white line is widest where the heel joins the wall. During weight bearing there is widening of the white line and greater exposure of the soft flexible horn to the bearing surface resulting in increased wear. Dirt and bacteria get trapped into the separated area and may form an abscess which may extend into deeper tissues of the foot. Causes of white line disease include:
1. Mechanical shearing caused by rough flooring;
2. Biomechanics of weight bearing: pigs walking with a ‘swivel gait’ in the hind quarters displace more weight onto the outside claws;
3. Inflammation of the corium caused by laminitis;
4. Bacterial infection of the white line causing further deterioration of the soft horn;
5. Poor horn quality caused by deficiencies or laminitis.
Horn – growth and wear
In a study on horn growth and wear, conducted by myself and published last year in the Journal of Swine Health and Production, the growth rate was found to be greater for back feet (7.42 mm/month) compared to front feet (5.14 mm/month). Similarly the rate of wear was greater for back feet (6.26 mm/month) compared to front feet (3.97 mm/month). Compensatory growth was thought to compensate for the higher rate of wear.
Overall growth rates for the front (cranial) and back (caudal) wall segments were found to be 5.48 mm and 6.98 mm/month respectively. The mean wear rate for the cranial and caudal wall segments was 5.03 mm and 5.25 mm/month respectively. This may indicate more weight bearing on the caudal aspect of the wall thus more wear and growth. Overall mean growth rate for the inner claws was 6.71 mm/month and outer claws 5.81 mm/month. Toe length increased over the study period except for the left front inner claw which decreased. Claw surface was similar between front claws whereas there was a big difference between claws in the back feet. The outer claw of the back foot is generally much bigger than the inner claw indicating that the outer claw in the back foot carries more weight compared to the inner claw.
 Beheer
Beheer








 WP Admin
WP Admin  Bewerk bericht
Bewerk bericht